Cathodic Protection: The Ultimate Guide to Corrosion Prevention for Metal Surfaces
Corrosion is a natural process that can cause significant damage to metal surfaces over time. From pipelines and tanks to ships and infrastructure, the effects of corrosion can be costly and even dangerous. Fortunately, there is a solution: cathodic protection. This innovative technique uses sacrificial anodes or external power sources to protect metal surfaces from corrosion, prolonging their lifespan and saving you money in the long run. In this guide, we’ll explain the science behind cathodic protection and how it’s used in various industries. We’ll also cover the importance of regular monitoring and maintenance for effective results. Whether you’re a facility manager, engineer, or just curious about corrosion prevention, this guide has everything you need to know about cathodic protection.
Cathodic protection is a method used to prevent corrosion of metal surfaces by making them the cathode of an electrochemical cell.
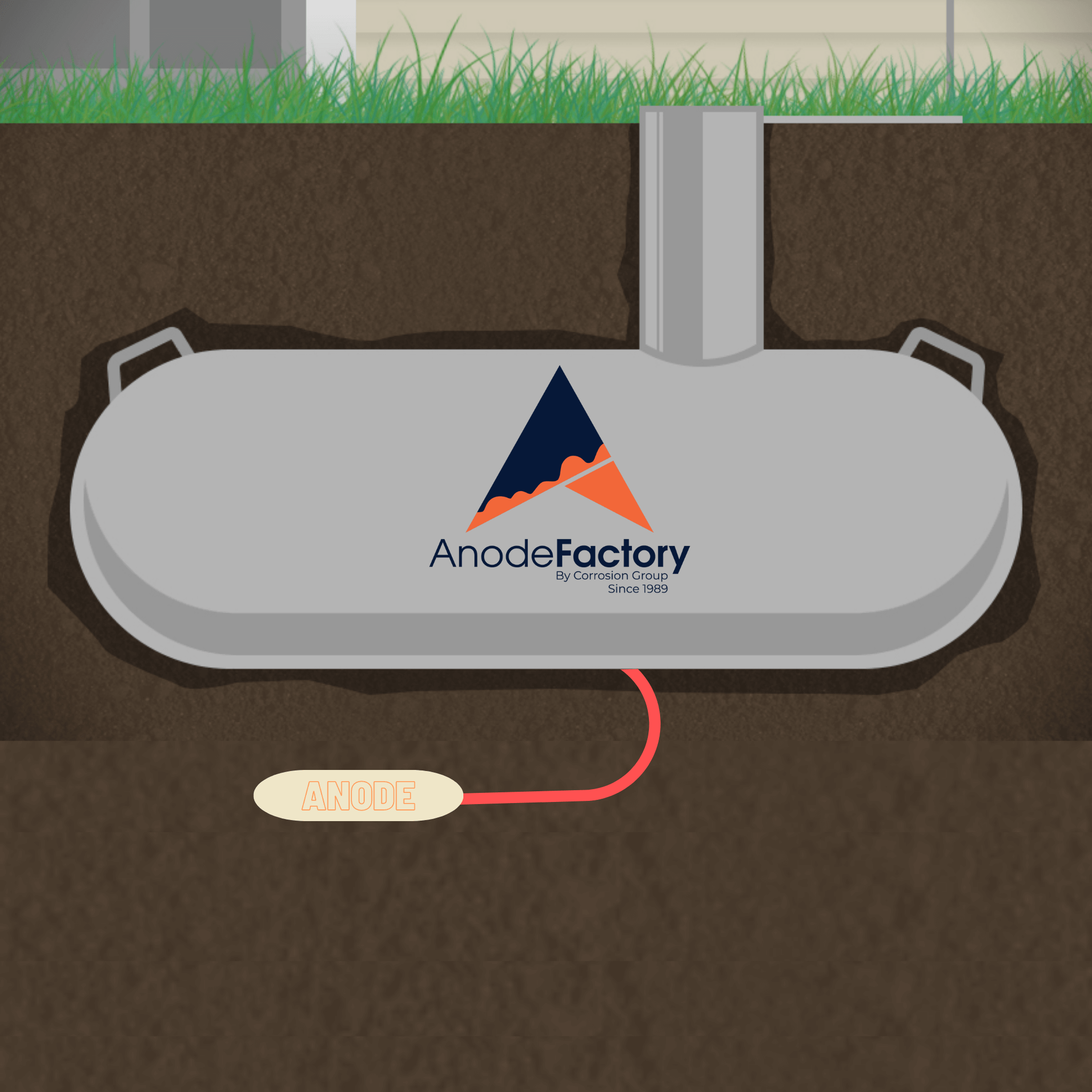
This is achieved by connecting the metal surface to be protected to a more easily corroded “sacrificial metal” that acts as the anode of the cell. The sacrificial metal corrodes instead of the protected metal, thus protecting it from corrosion.
The most common method of cathodic protection is to bury a more easily corroded metal, such as zinc, aluminum, or magnesium in contact with the metal surface to be protected. This is known as sacrificial anode cathodic protection.
Another method is to use an external power source to drive an electric current through the metal surface to be protected, thereby creating an artificially induced electrochemical cell in which the metal surface is the cathode. This is known as impressed current cathodic protection.
Cathodic protection is widely used in a variety of industries, including oil and gas, marine, and infrastructure. It is particularly effective for protecting buried or submerged metal structures, such as pipelines, tanks, and ships.
To ensure the effectiveness of cathodic protection systems, regular monitoring and maintenance is essential. This includes monitoring the potential (voltage) and current of the system, as well as the condition of the sacrificial anodes or impressed current rectifiers.
Overall, Cathodic protection is a cost-effective method of protecting metal surfaces from corrosion, and it is widely used in various industries for the preservation of metal assets.