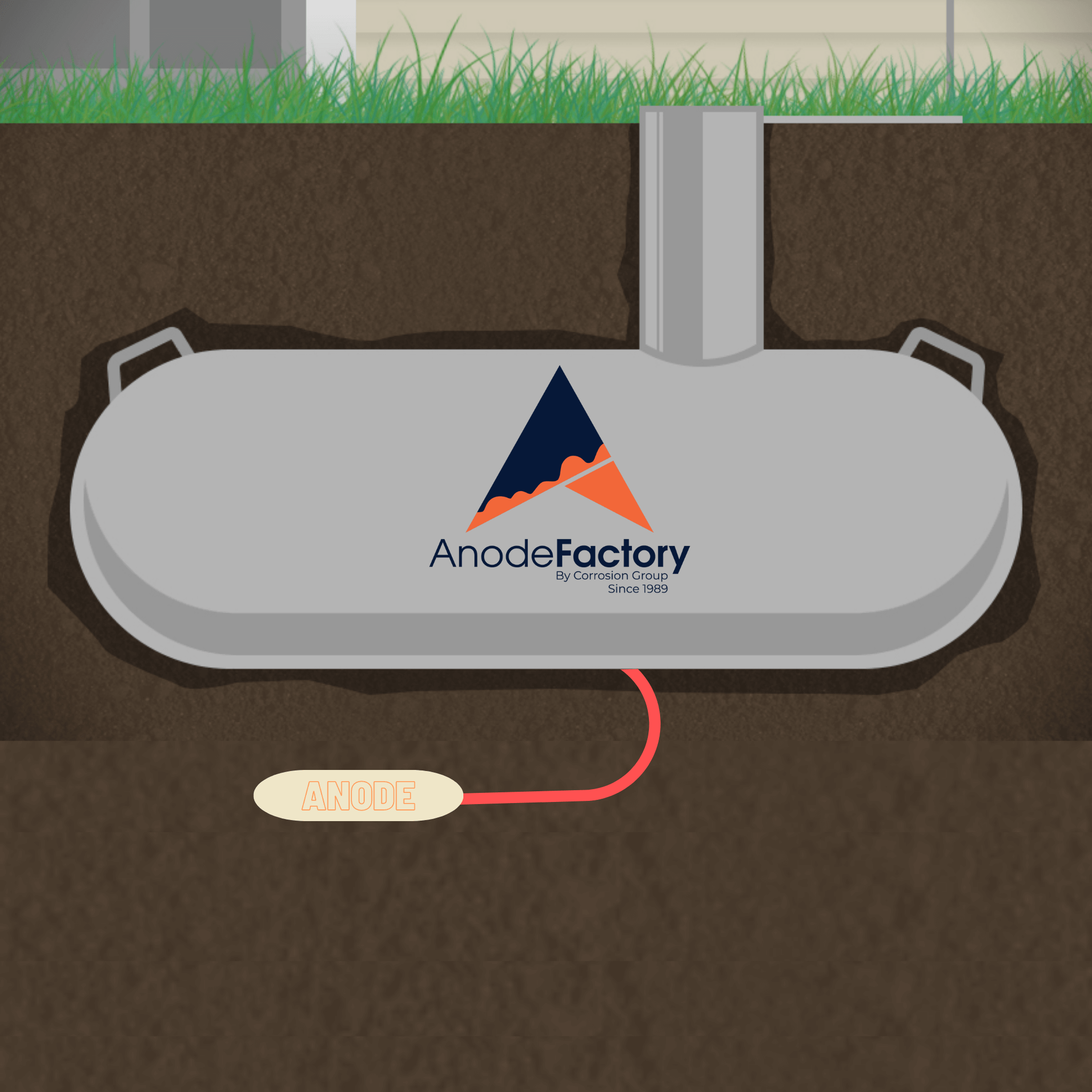
Cathodic Protection below the waterline (ship hulls)
In this post we take a “deep dive” into cathodic protection below the waterline. Primarily regarding the hull of ships and vessels.
It is almost impossible to cathodically protect a hull that is not coated, due to protection current requirements and current distribution.
In addition to this, there must be an electrically insulating layer between the steel wall and the anti-fouling coating in order to stop an electro-chemical reduction of toxic metal compounds.
There is a clear difference between complete and partial protection of a underwater area, which depends on the extent of the protected object.
Partial protection usually involves protecting the stern of the boat. This is done because of high flow rates, aeration and the formation of cells on attachments (such as the propeller and rudder ) .
Partial protection can also be extended to the bow, which also is subject to high rates of flow.
Complete protection of ships with galvanic anodes or impressed current is becoming more and more important. Since damage to the coating due to incidental mechanical damage can be quite frequent.
In all cases, partial or total hulls of aluminium or stainless steel must be protected by cathodic protection. High alloy steels with over 20% chromium and 3 % molybdenum are very prone to corrosion beneath the coatings.
We hope this has made things much more clearer!
Note: We experience many customers stating that they have been recommended to use Aluminium anodes more and more often. We want to put out a friendly reminder that: The choice of anodes is not only based on what type of anode is more durable or not. Several other factors come into play like the type of water (freshwater, marine), the total surface of the hull and so on.
Please get in touch if you want to know more.