Cathodic corrosion protection – with sacrificial anodes, providing corrosion protection
Cathodic Protection – with sacrificial anodes, providing corrosion, the ultimate corrosion protection? Probably.
The real question however is, can we by applying complete cathodic corrosion protection, increase the design life of steel structures substantially, for how long, at what cost? Allow us to elaborate…
What is cathodic corrosion protection with sacrificial anodes and how does it work?

Cathodic protection (CP) is a technology used to control and prevent the corrosion of a metal surface by making it the cathode of an electrochemical cell. Cathodic corrosion protection (CP) moves corrosion away from the protected structure and toward the less noble metal (the sacrificial anode). Cathodic protection in principle can be applied to any metal structure that comes into contact with an electrolyte (e.g., water, soil or concrete), though its primary application is to protect steel structures that are either embedded in the ground or submerged.
Cathodic protection is generally used to protect several structures from corrosion, such as ships, offshore infrastructure, underwater machinery, harbors, pipelines, tanks; essentially any submerged or buried metal structures. The cathodic protection technology for steel keeps the metal safe and guarded from corrosion. In most cases its widely know that protecting structures with cathodic protection, you can increase the design life of the structure by a factor of 10!
Here is a short explainer video of how cathodic corrosion protection works and some areas of usage:
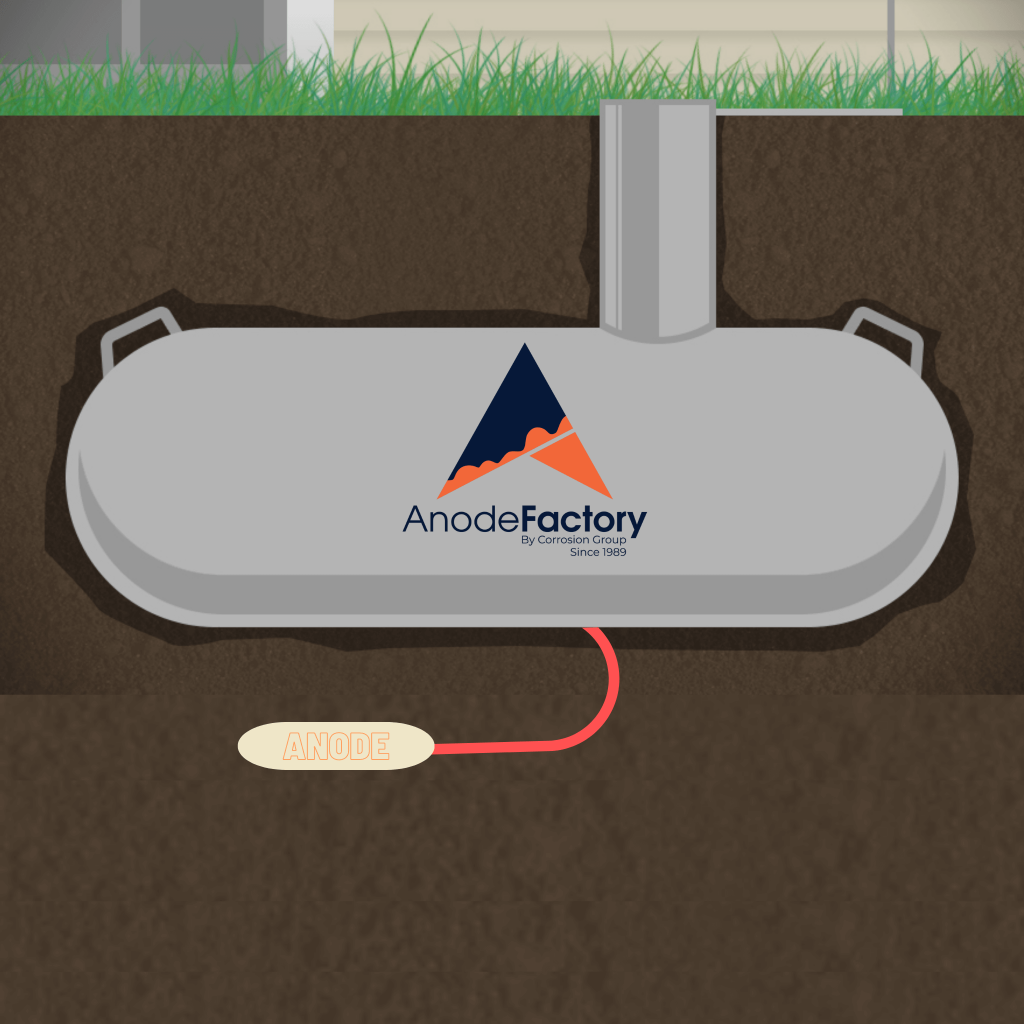
Cathodic protection example, using sacrificial anodes. These sacrificial anodes are often referred to as Magnesium anode bags.
In cathodic protection with sacrificial anodes, metals with a more negative electrode than steel is used, than the structure they shall protect. The anodes consist of zinc, aluminum or magnesium, depending on the conditions. The sacrificial anode is connected to the pipe, which causes a positive current to flow from the anode into the pipe, such that the entire steel surface becomes more negatively charged, which is a cathode. Because of the potential difference between the anodal area (less noble) and cathodal area (steel), the positively charged metallic ions exit from the anodal surface, and the electrons exit the cathodal surface. By electrically connecting the metal being protected with the most anodic (electronegative) metal, we ensure the anode will sacrifice itself by preferentially corroding to its cathodic counterpart. The latter metal, commonly called the galvanic or sacrificial anode, has less negative electrochemical potential than a piece of regular steel. Hence, the main reason the more positive metal will obtain protection.
Commonly used to protect several structures, such as pipelines, ships, tanks, and offshore oil platforms, cathodic protection works by allowing the more reactive, sacrificial metal to corrode in place of the protected metal. This way, you control the corrosion process actively. Not passively, which you do with a coating or a paint.
The water & gas distribution alongside with the oil and gas industry uses cathodic protection systems to prevent corrosion of pipelines, steel storage tanks, offshore platforms, and well casings. Benefits are many.

Read more about corrosion and its preventative measures?
- Cathodic Protection
- Community
- Concrete Corrosion Structures
- Corrosion in Harbours
- Corrosion on Ships
- News
- Uncategorized
Anodes Cathodic Protection Corrosion Corrosion On Ships Corrosion on water distribution lines Corrosion Prevention On Boats Corrosion Protection for Boats Magnesium Magnesium anode bag Ships Water distribution lines Zinc


